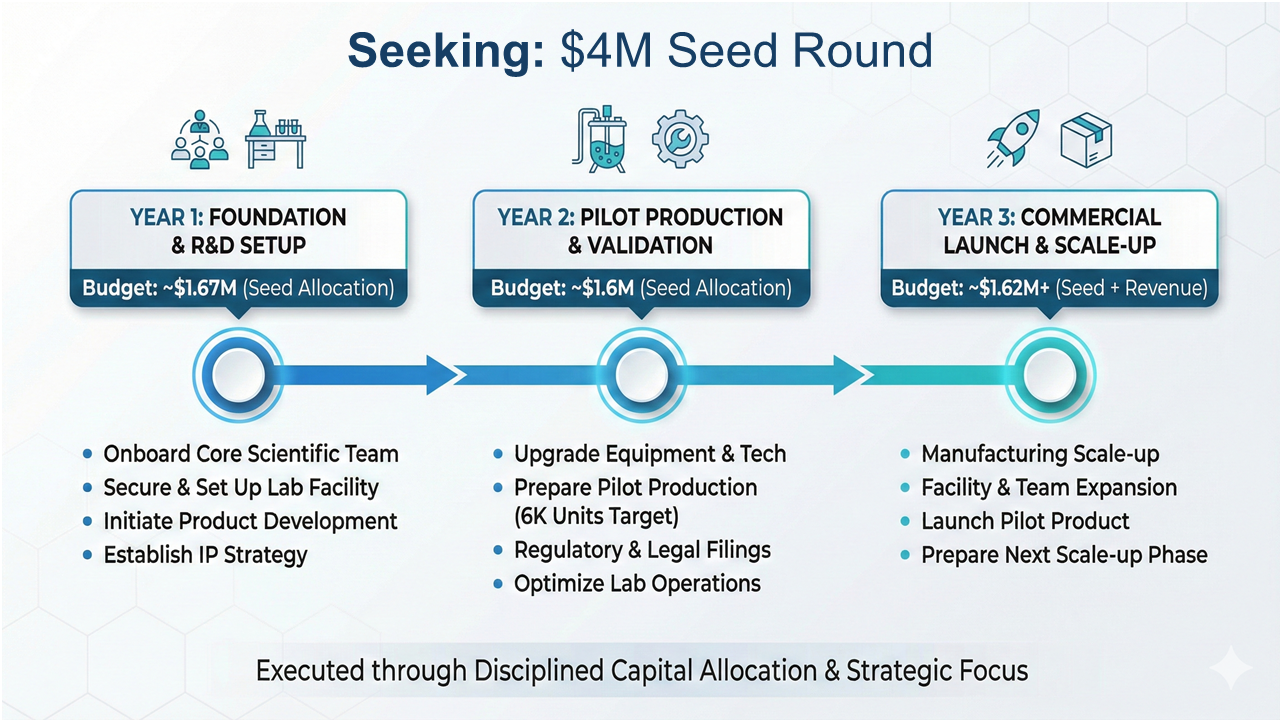
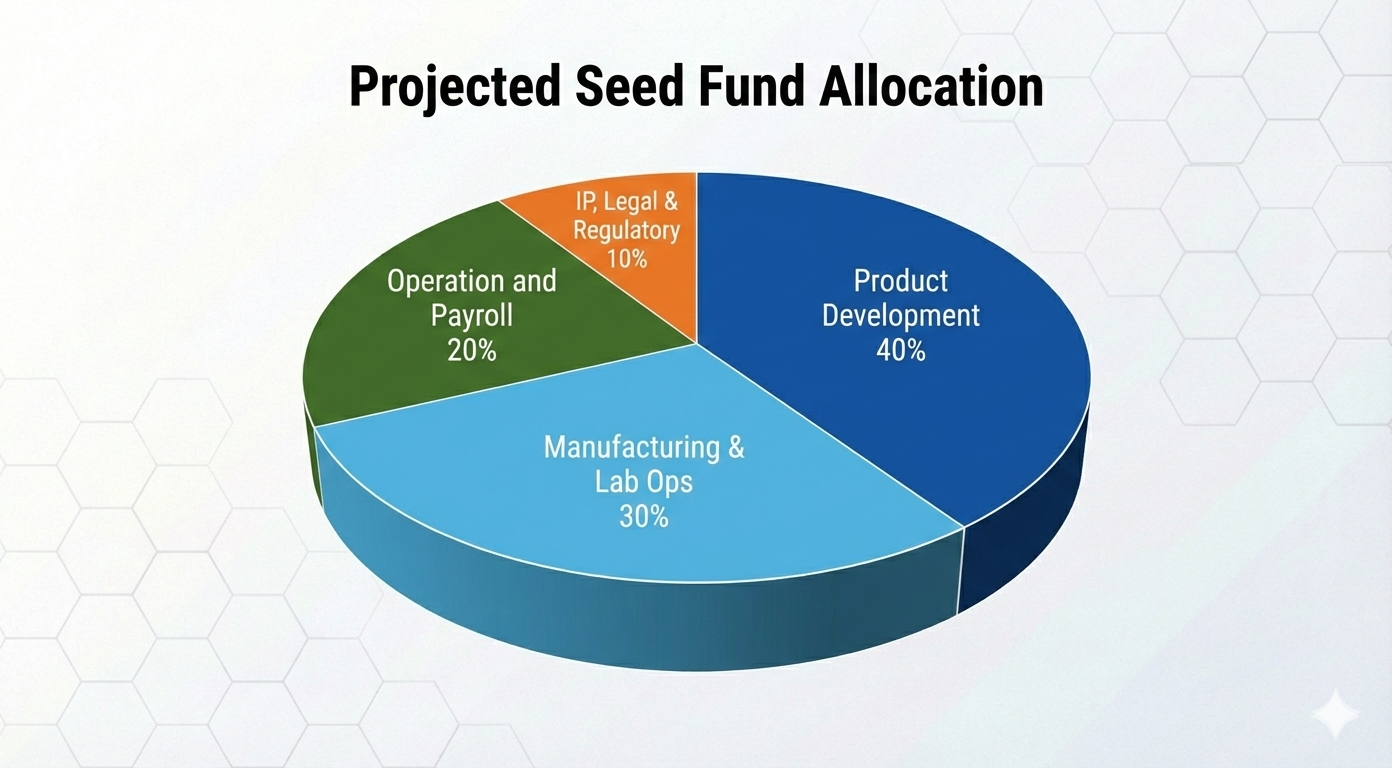
Investing in the Scalable Future of Cellular Medicine
With the global regenerative medicine market projected to reach $48.83 billion by 2034 (Precedence Research), Chiron is uniquely positioned to capture significant market share—delivering both near-term impact and long-term value.
This strong foundation of technical credibility, IP defensibility, and a clear market‑entry path positions Chiron for substantial upside in a rapidly expanding sector.
$12.35 billion market (2023) is projected to grow at a CAGR of 11.2% by 2030, positioning Chiron to lead the adult stem cells market.
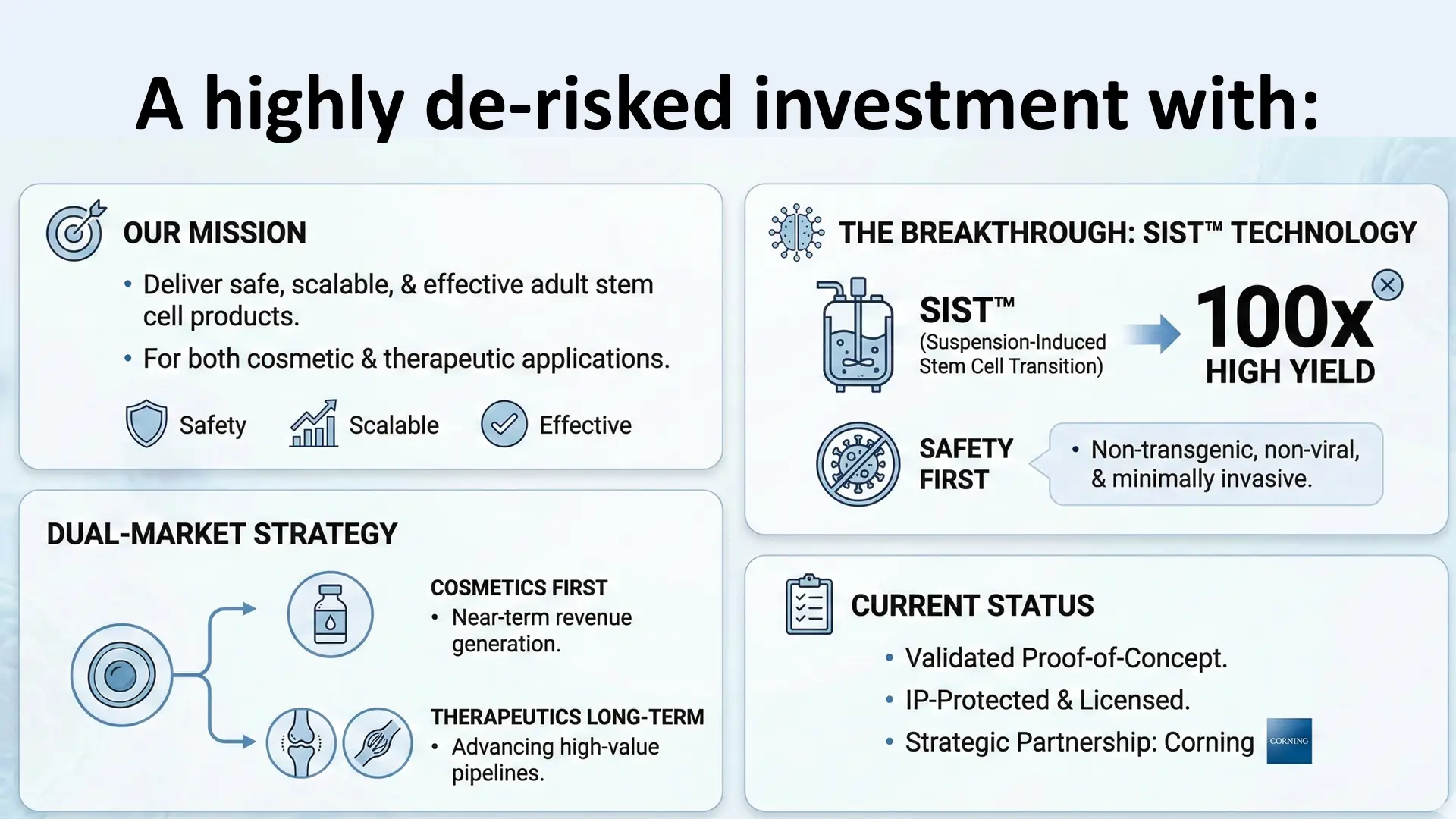
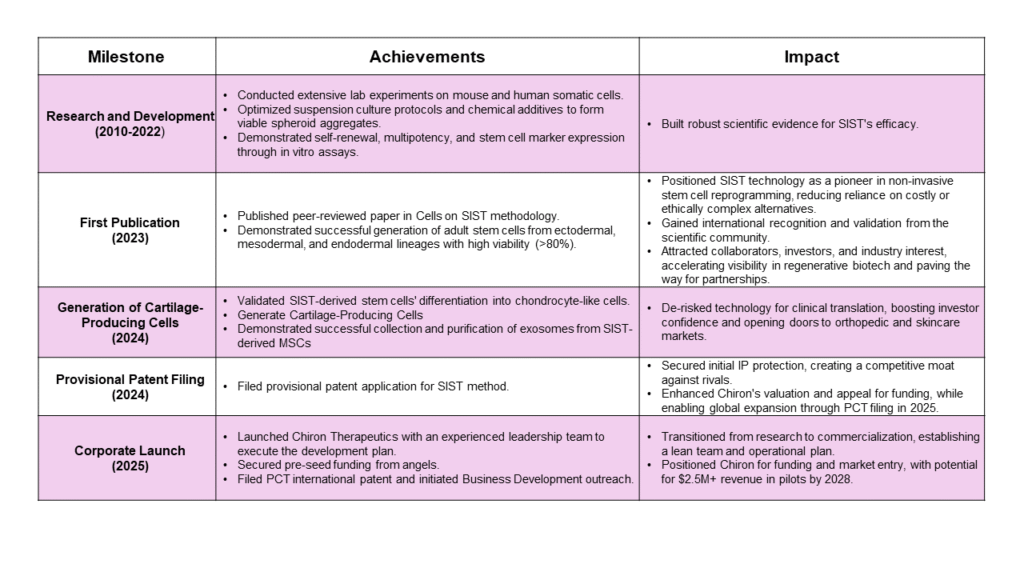
The investment is supported by a proven, non‑transgenic SIST method for high‑yield, scalable adult stem cell production, protected by a secured PCT international patent filing, with ongoing enhancements to optimize exosome purity and yield.
A strategic "cosmetic ingredients" focus with no therapeutic claims for faster market entry with lower regulatory hurdles
The rapidly growing global regenerative medicine and aesthetics markets present a substantial opportunity for Chiron's innovative technology.
Key Market Drivers
Rising demand for non-invasive, science-backed solutions among aging populations worldwide. Asia Pacific shows fastest growth potential.
Chiron Therapeutics is pioneering a new era in skincare and skin rejuvenation through stem cell–derived exosomes.
The adult stem cells market is projected to reach $12.4 billion by 2023, with steady growth to 2030 at a CAGR of 11.2%. This trend highlights the increasing demand for regenerative medicine solutions like those offered by Chiron Therapeutics.
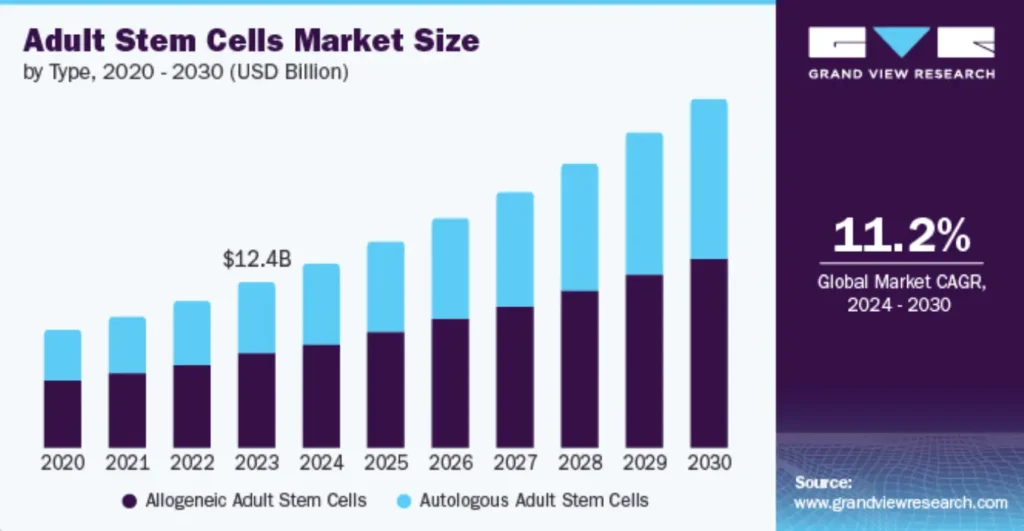
Global Market CAGR, 2024-2030
Fastest growing segment in the market
Chiron's SIST™ technology offers a clinically validated, transgene-free solution to the expansion of adult stem cells—100x more efficient than traditional methods—while avoiding the complex FDA hurdles of iPSC-based therapies.
Avoids iPSC FDA hurdles (e.g., tumorigenicity risks delaying approvals) by using transgene-free approach with adult stem cells
100x expansion of adult stem cells enables cost-effective manufacturing at scale, addressing the massive gap in clinical demand
Strategic Collaboration with Corning

Corning will be providing support via the supply of Elplasia plates for adult stem cell manufacturing.
Enables us to optimize the scale up of SIST based production of adult stem cells. They cost-effectively drive Chiron's ability to manufacture adult stem cells at scale.

Critical for preclinical data generation and eventual FDA/Health Canada filings.

Dr. Behzad Yeganeh is the visionary Founder and CEO of Chiron Therapeutics, a seasoned biotech leader with over 15 years of experience translating complex biological discoveries into commercially viable assets. A two-time founder and serial entrepreneur, Dr. Yeganeh combines deep technical expertise in molecular biology with a proven track record of launching successful ventures anchored by proprietary intellectual property.
As the inventor of the revolutionary SIST technology, Dr. Yeganeh achieved a breakthrough high yield in generation of adult stem cell without genetic modification. This IP-licensed invention positions Chiron Therapeutics at the forefront of regenerative innovation, unlocking transformative therapeutic and cosmetic applications for adult stem cells.
Dr. Yeganeh’s leadership extends from bench to pre-clinical validation, including extensive experience in GMP manufacturing and regulatory strategy, notably with Health Canada. He has demonstrated a strong ability to secure non-dilutive funding and drive R&D strategies that bridge the gap between scientific innovation and market reality.